Magnesium – a promising but inflexible candidate
Magnesium is the lightest metallic construction material. Its density is about 60 percent that of aluminum, making it a significant material for lightweight construction. In addition, magnesium is one of the most abundant elements in the earth’s crust. The melting range of magnesium is between 430 °C and 630 °C, which facilitates processing. Another important aspect for the use of magnesium and its alloys is their high recyclability.
Despite these advantages, magnesium alloys have only a small market share today. One of the reasons for this is the limited formability of magnesium and its alloys. One measure of the formability of the materials is the number of slip systems present. The higher the number of slip systems, the easier it is for dislocation movements to take place in the metallic material. Due to its hexagonal lattice structure, magnesium has only three slip systems, while aluminum and steel alloys each have twelve slip systems. During plastic deformation, the crystal lattice changes and atoms move to new places as dislocations move. In the cubic crystal structure of aluminum, this is possible via many paths. For this reason, sheets of aluminum can be deep-drawn comparatively easily into almost any shape. Magnesium atoms, however, arrange themselves in a hexagonal lattice, so that the possibility for dislocation movements is correspondingly severely limited.
Possible applications from engines to medicine
For this reason, magnesium alloys are nowadays mainly used as a structural material for die cast components such as engine and transmission housings (see Fig. 1) in the automotive industry and in aircraft construction. Due to remarkable weight savings, this enables energy-efficient flights as well as increased payload. In addition to the weight reduction aspect, magnesium alloys have excellent damping properties. As a result, vibrations and noise emissions in the engine can be significantly reduced.
Magnesium alloys are also used in modern medicine. As screws and plates, for example, they stabilize complicated bone fractures or are used as stents in vessels, for example after a heart attack. The decisive advantage of magnesium in such applications is its resorbability. This means that the metal is broken down and absorbed by the body over time, so that follow-up operations to remove the materials after healing are no longer necessary.
Opening up new possibilities through research and development
If scientists succeed in developing methods for simple forming of magnesium alloys, the wide range of excellent properties of these materials can be extended to other industries and areas of application. The use of the so-called electroplastic effect (EPE) could be one way to improve the formability of such attractive materials and thus expand the range of applications.
The EPE is a phenomenon that occurs due to many very small, simultaneous physical effects when a high electric current is introduced in metallic materials during forming. In general, EPE facilitates the plastic forming of metals. So far, it is not clearly understood which of the many physical effects has the largest and most important influence on formability. A targeted industrial application of EPE requires an in-depth understanding of the mechanisms involved. The challenge here is that the effects overlap and are difficult to identify.
The hurdles of analytical methods
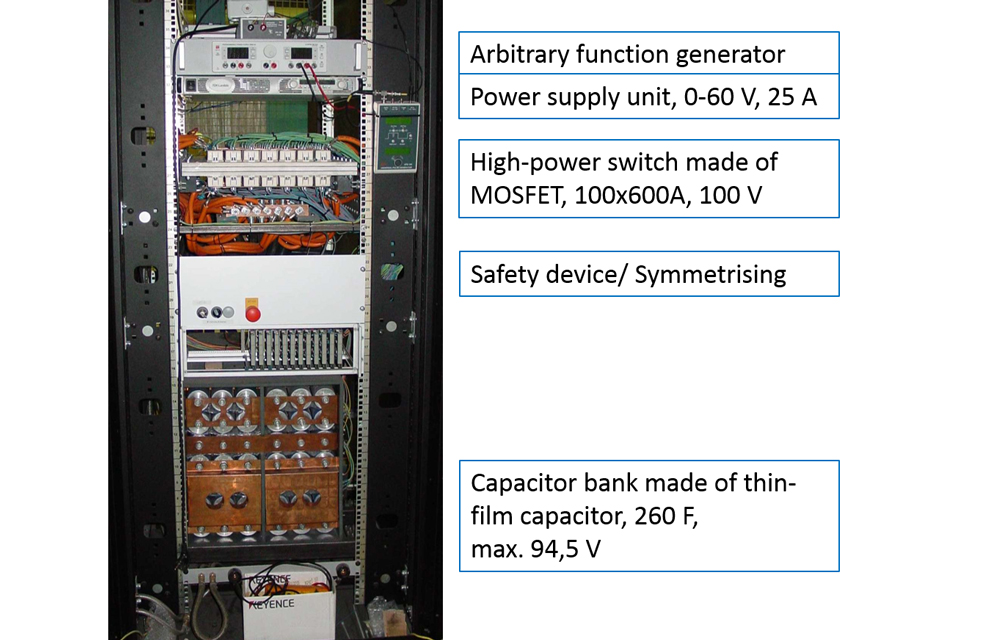
Scientists at the Institute of Materials Science (IW) at Leibniz Universität Hannover are investigating selected alloys with lightweight potential for this purpose by applying high current pulses during a compressive and three-point bending load. To characterize the mechanisms involved, a whole range of further methods are needed to analyze microstructure evolution.
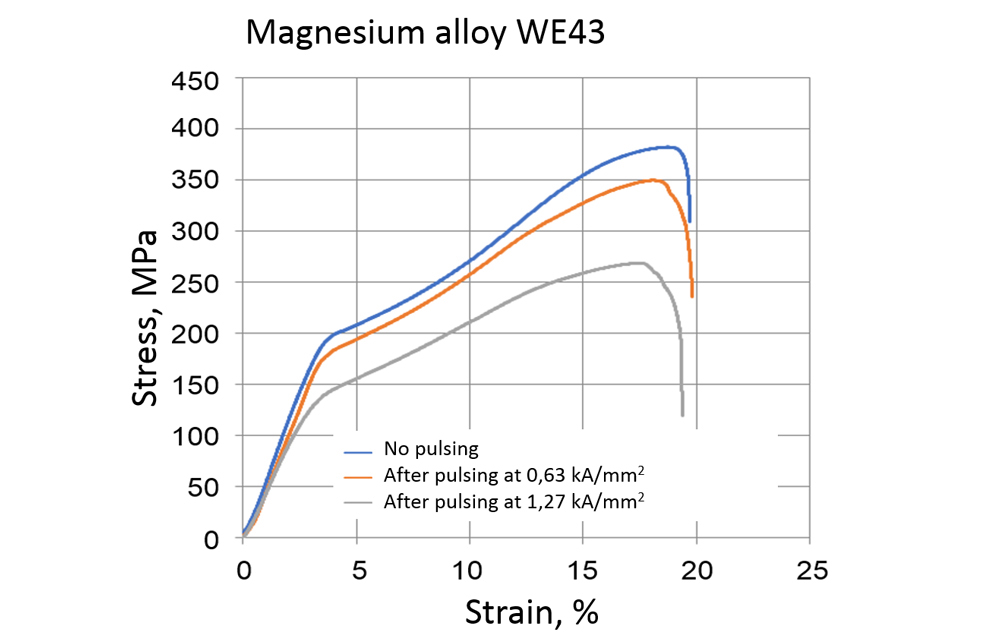
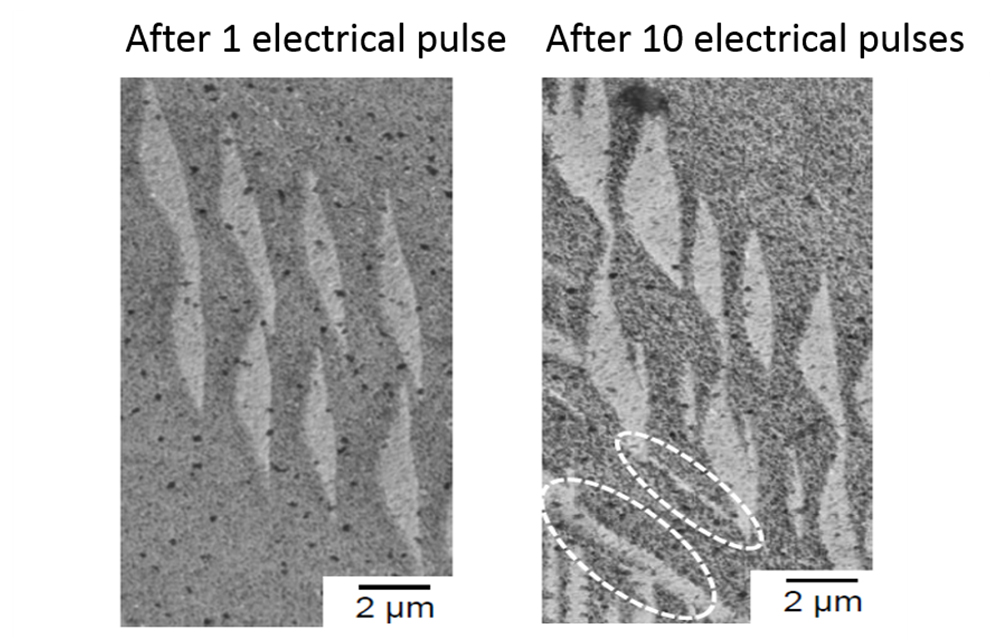
For example, the influence of current pulses on the forming behavior of the alloys is characterized by comparing stress-strain curves with and without current pulses (see Fig. 3). The results clearly show that significantly lower forces are required to achieve the same compression when compressive loading is combined with current pulses. In order not to misunderstand the results, the researchers must pay attention to all influencing factors – such as the heating of the specimen as a result of the current pulses. For an in-depth understanding of the mechanisms at the microstructural level, the samples are analyzed by electron microscopy, for example with regard to the development of selected microstructural features such as the formation of twin structures as a result of repeated current pulses (see Fig. 4).
The challenge in these investigations is to use highly sensitive methods to identify those changes that occur as a result of the EPE while considering sufficiently large data sets, and to use these specifically to achieve the desired goals. The researchers at IW have already mastered the first steps and identified areas at the microstructural level, that can be particularly well influenced by EPE. Accordingly, the next step can be a targeted adjustment of the microstructure for an increased reaction of the alloys to the current pulses.